Formulation Development
- Select
Home | Services & Solutions | Drug Product | Oral | Formulation Development
Formulation Development, Early Phase
Our oral solid formulation development teams have rich experience in accelerating your drug candidates from preclinical to clinical and commercial manufacturing.
We have tackled challenges ranging from poor bioavailability and excipient incompatibility to vehicle stability and optimal release profile. We are now supporting 10+ commercial drug products for the global market.
Covering a wide range of oral dosage forms, we have established a comprehensive enabling technology platform that includes spray dried dispersion, hot melt exclusion, nano-suspension and lipid formulation.
We also provide support for the development and manufacturing of high-potency oral drug formulations, with an Occupational Exposure Limit (OEL) ≥ 10 ng/m³.
Speed
- 1-6 weeks for formulation development
- 4-6 weeks from GMP API received to phase I CTM released (F2CS)
- 6 months from technology transfer to release of PPQ completed (F4CL)
Experience
- 1,000+ preclinical-phase 3 molecules supported in 2024
- 2,600+ batches manufactured in 2024
- 14 commercial projects supported
Enabling Technology Platform
Speed
- 4-6 weeks from GMP API received to phase I CTM released (F2CS)
- 1-6 weeks for formulation development
Experience
- 2,500+ compounds assessed annually
- 300+ formulations developed annually
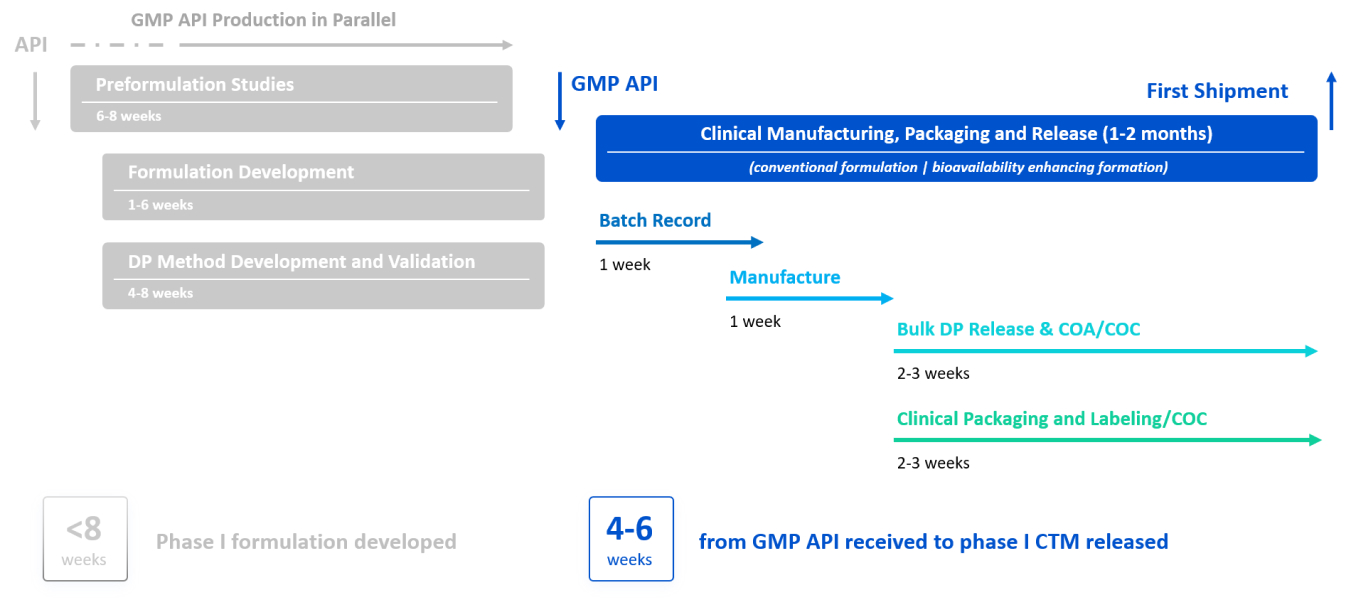
Fast to Clinical Supply (F2CS) Service Package
Drug Product Service Package Designed to Deliver CTM for Phase I Clinical Trial Fast
Your innovative therapy is ready to reach a key milestone – the IND submission. When the date quickly approaches, you need a reliable partner to formulate and manufacture your therapy to meet the filing requirements and the dosage needs for phase I studies fast.
Every innovation is unique, and so your partner needs to offer the right mix of formulation and dosage form options and rapidly use data-driven models to determine the best option – not a one-size-fits-all mindset.
WuXi STA’s F2CS package applies our capability, expertise, and advanced technologies to deliver your clinical trial material fast. F2CS is our promise of formulation development and CTM supply both within 8 weeks.

Why trust F2CS?
WuXi STA teams are highly experienced, supporting 1,000+ preclinical-phase III molecules in 2024 in different dosage forms. Our teams develop appropriate formulations utilizing a variety of dosage forms including a complete bioavailability enhancement toolbox for fast delivery of CTM.
Fast – Our integrated drug substance, drug product, analytical, and CMC writing teams work in parallel to save time and complete your project fast. Our large team and facility capacity are able to accommodate unexpected changes fast to ensure on time and on budget delivery.
Flexible – Our teams are experienced with a wide variety of dosage forms and equipped with multiple processing tools. They have access to the most advanced bioavailability enhancing technologies enabling the selection and design of a dosage form that best achieves optimal exposure.
Integrated – Drug product and analytical teams are located at the sites within 2 hour drive from drug substance teams, making collaboration, problem-solving, scheduling, and material transfer fast and easy.
Bioavailability Enhancement Technologies
- Spray Dried Dispersion
- Hot Melt Extrusion
- Nano Suspension
- Lipid Formulation
Flexible Formulation & Process Options
- API in Bottle or Capsule
- Powder in Capsule
- Tablets
- Lipid Formulation
- Roller Compaction
- Direct Compression
- Wet Granulation
Integrated CMC Services
- API Process R&D and Manufacture
- Analytical Development & Validation
- Stability Testing
- Packaging and Labeling
- CMC Writing
- Proven Quality and EHS System
Fast to Clinical Supply
Speed
- Technology transfer to release of PPQ completed in 6 months (F4CL)
Experience
- 500+ molecules in Phase II and beyond (2022)
- 2,800+ clinical and commercial batches manufactured (2022)
- 8 commercial projects
Fast for Commercial Launch (F4CL) Service Package
Drug Product Service Package Designed for Fast Commercial Launch to Global Markets
When your CMC projects approach the final step to commercial approval – NDA submission, you need to consider how to ensure your drugs successfully reach your intended markets.
Regulatory agencies have different requirements and timelines. Working with the right partner, and so you don’t need to worry about the markets you are not familiar with. WuXi STA has extensive experience in all CMC activities and project planning for global filings.
Our dedicated drug product service package – Fast for Commercial Launch (F4CL), can support your commercial launch in global markets in parallel with speed, reliability and quality.

Why trust F4CL?
WuXi STA has a large team of formulation scientists and analytical scientists across North America, Europe and Asia to support design and implementation of your global commercialization goals.
Speed
Our integrated API and drug product CMC platform has large teams offering high capacity and flexibility to customize plans and run in-parallel programs whenever possible to reduce time to submission.
Capability
Our formulation teams have broad dosage form capability supported by state-of-the-art facilities equipped with enabling technologies including spray dried dispersion and hot melt extrusion to support your selected formulation.
Experience
Our integrated teams have deep experience and know-how delivering late stage CMC campaigns supporting 14 commercial products.
Reliability
Our integrated CMC platform currently supports more than 1,000+ drug from preclinical to phase III every year. Globally our products have been launched in more than 105 countries.
Quality
Every facility is managed by a consistent quality system with a proven track record of approvals from all major regulatory agencies and hundreds of client audits every year.
Related Resources

Spray Dried Dispersion from Preclinical to Commercial

Pave an Expressway to Phase I – Strategies that Accelerate Your Early Phase Programs
Formulation Development, Late Phase
Our dedicated late phase teams have rich experience in pushing your candidates from early stage to pivotal clinical, process optimization via DoE, process validation, and NDA registration, until commercialization.
We have supported validation of 20+ NCEs since 2019 with 100% PPQ success, and have successfully enabled 7 drug product approvals for the global market. Our GMP facilities in Shanghai, Wuxi city, China and Couvet, Switzerland have been inspected by the US FDA, EMA, China NMPA, Japan PMDA, SwissMedic and many other regulatory agencies, equipped with advanced commercial equipment for spray dried dispersion, hot melt extrusion, dry/wet/fluid bed granulation, continuous manufacturing, high potency drug product and many more.