Drug Product

We have a formulation R&D team of 1,500+ formulators & analytical scientists, providing formulation development and manufacturing from preclinical to commercial phase. Our integrated drug product R&D and manufacturing facilities around the world supports both oral solid and parenteral dosage forms with various packaging formats.
Bioavailability enhancing technologies such as lipid nanoparticle and spray dried dispersion are also available from laboratory to production scale.
In 2024 alone, 1,000+ preclinical-phase III molecules were supported. Till now, 50+ late-phase NCEs were validated , and we supported 10+ commercial products.
Drug Product Services
Oral
Parenteral
Analytical
Packaging, Labeling and Distribution
High Potency
Enabling Technology Platform
Why WuXi STA?






Dont edit
Speed
8-12 weeks for IND ready formulation, 4-6 weeks from GMP API received to phase I CTM released, technology transfer to release of PPQ completed in 6 months
Team
1,400+ formulators and analytical scientists
Global Quality Standard
One quality system across all sites approved by major regulatory agencies in the world
Robust Supply
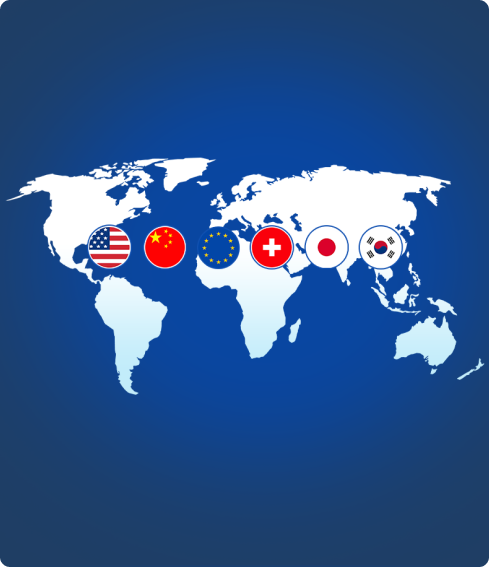
4 sites in United States, Europe and China support large scale production.
Integrated Collaboration
Formulation R&D – early phase, late phase, commercial manufacturing and analytical & QC teams work closely to streamline your projects
Enabling Technology
Lipid Nanoparticle, Spray Dried Dispersion, Hot Melt Extrusion enable your fomrmulations with enhanced solubility.
5 Drug Product Sites Globally
Featured Service Packages

Fast for Commercial
Launch
(F4CL)
Technology transfer to release of PPQ completed in 6 months

IND Enabling Pre-Formulation Package (IEPP)
IEPP is an integrated developability assessment service for new chemical entities (NCEs) to efficiently identify challenges and solutions earlier for higher success rates and lower overall costs. It usually takes 8-12 weeks for IND ready formulation.

Fast to Clinic Supply
(F2CS)
F2CS package applies our capability, expertise and advanced technology to deliver effective clinical trial material fast. F2CS is the abbreviation of “Fast to Clinical Supply”, representing the promise of formulation development and CTM supply both within 8 weeks.

Fast for Commercial
Launch(F4 CL)
Our dedicated drug product service package – Fast for Commercial Launch (F4 CL), can support your commercial launch in global markets in parallel with speed, capability, experience, reliability and quality.