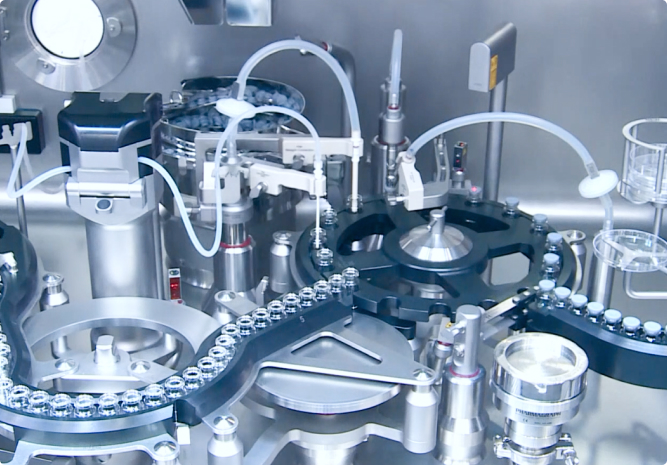
制剂
制剂服务
口服制剂
非口服制剂
Analytical
Packaging, Labeling and Distribution
High Potency
Why WuXi STA?






Dont edit
Speed
8-12 weeks for IND ready formulation, 4-6 weeks from GMP API received to phase I CTM released, technology transfer to release of PPQ completed in 6 months
Team
1,400+ formulators and analytical scientists
Global Quality Standard
One quality system across all sites approved by major regulatory agencies in the world
Robust Supply
4 sites in United States, Europe and China support large scale production.
Integrated Collaboration
Formulation R&D – early phase, late phase, commercial manufacturing and analytical & QC teams work closely to streamline your projects
Enabling Technology
Lipid Nanoparticle, Spray Dried Dispersion, Hot Melt Extrusion enable your fomrmulations with enhanced solubility.